Biomanufacturing
We are an innovative biomanufacturing company in Canada that uses advanced technologies and engineering approaches to automate the production of nanotechnology diagnostic products in a more efficient, cost-effective, and scalable way.
Our approach encompasses genetic engineering, cell culture, fermentation, and downstream processing to optimize the entire production process from design to commercialization.
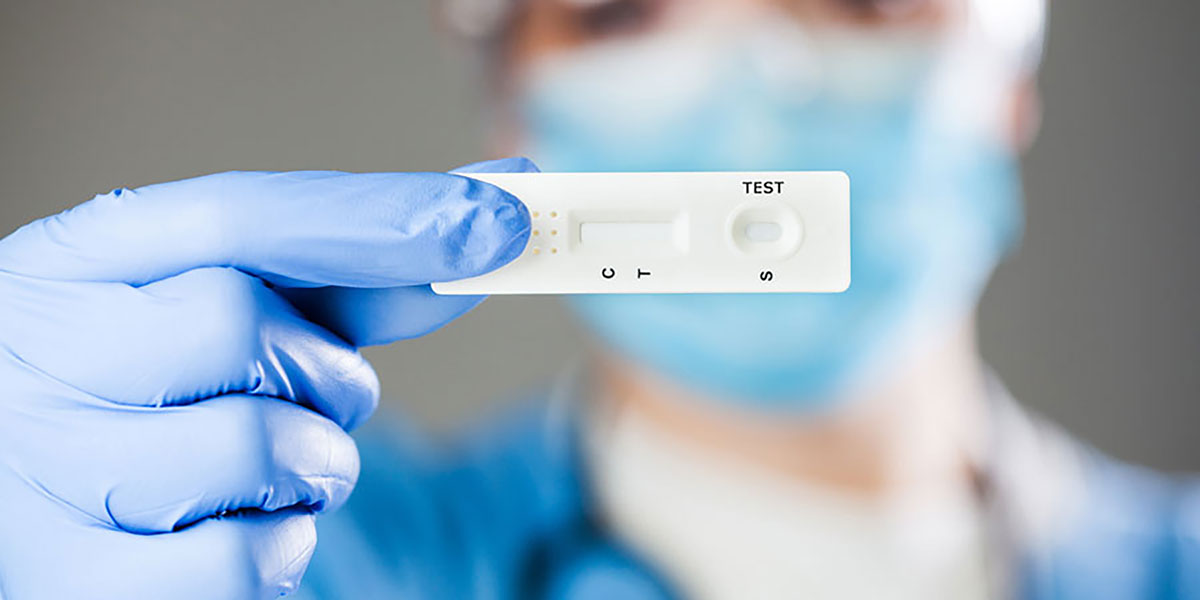
A lateral flow immunoassay is a user-friendly diagnostic device that is commonly used to determine the presence or absence of a specific substance, such as pathogens, biomarkers, or contaminants. These tests are employed in various fields, including human and animal health, environmental testing, food and feed analysis, and plant and crop health.
Lateral flow immunoassays, often known as rapid test strips, typically feature a control line to ensure proper functioning and one or more target or test lines. They are designed to be intuitive for users and require minimal training to operate. These tests can provide qualitative visual results or offer data when used with reader technology. Notably, the most well-known type of lateral flow rapid test strip is the pregnancy test.
| Lateral flow test (LFT) | Express test |
| Lateral flow device (LFD) | Pen-side test |
| Lateral flow assay (LFA) | Quick test |
| Lateral flow immunoassay (LFIA) | Rapid test |
| Lateral flow immunochromatographic assays | Test strip |
Lateral flow tests (LFDs) operate based on immunoassay technology, employing components such as nitrocellulose membrane, colored nanoparticles or labels, and antibodies to generate results.
When a sample is applied to the test device, it flows through the conjugate pad and onto the nitrocellulose membrane. Finally, it reaches the absorbent pad, completing the flow along the test device.
Regardless of the label types, they all perform the same function to create a three-way bond with antibodies and targets in order to make the control and test lines visible.
Diagnostic technology is rapidly advancing, particularly in antibody identification, in automated clinical chemistry laboratories. With a wide dynamic range, high sensitivity and specificity, quantitative results, automation, reduced turnaround time, and the ability to conduct multiple antibody tests on large antigenic panels, this technology is highly advanced in clinical laboratories. It significantly impacts workflow and the organization of autoimmunology labs.
Anticipated advancements in the coming years include the introduction of new analytical platforms like flow-injection chemiluminescent immunoassay, two-dimensional resolution for chemiluminescence multiplex immunoassay, and magnetic nanoparticles chemiluminescence immunoassay. These developments are expected to enhance the clinical efficacy of antibody tests.
Chemiluminescent analytical methods offer key advantages, including a wide dynamic range, high signal intensity, absence of interfering emissions (ensuring high specificity), rapid signal acquisition, stable reagents and conjugates, low reagent consumption, random access capability, reduced incubation time, and compatibility with immunology assay protocols. However, it is important to acknowledge that there are also some unforeseen limitations to be considered.
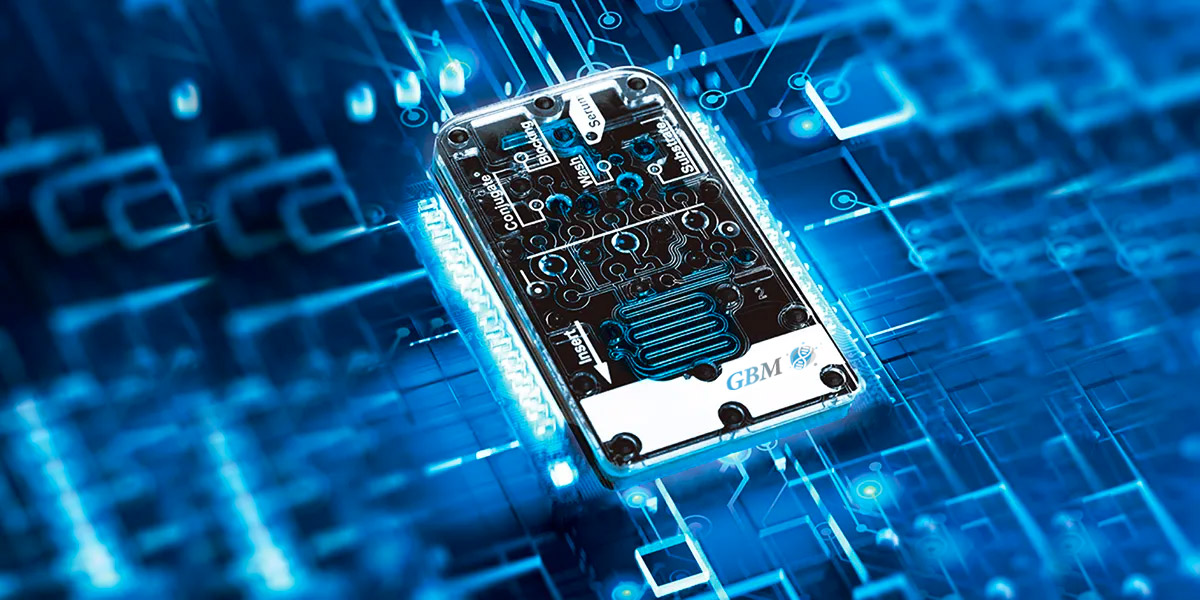
Gene Bio Medical’s microfluidic chip technology is an efficient, fast, accurate and automated latest detection platform technology that can miniaturize the entire reaction process onto a single chip.
The combination of microfluidic chip and high-sensitivity chemical light detection technology releases a series of high-throughput, multi-joint inspection, automation, low-pollution and high-accuracy diagnostic products.