From Pharmacy Shelves to National Milestone: SwiftSwab® Becomes First Canadian Brand with Formal Class IV Licence for COVID-19 & Flu Home Tests
Published Aug 30, 2025
SwiftSwab® Covid-19/influenza A&B Combo Home Test (License No.113844)
Canadian homegrown diagnostic brand SwiftSwab® proudly announces that its COVID-19 Antigen Home Test (License No. 113847) and COVID-19/Influenza A&B Combo Home Test (License No. 113844) have both received formal Class IV Medical Device Licenses from Health Canada【1】. This achievement marks another major leap forward for SwiftSwab®, following its initial entry into the market under emergency authorization (Interim Order, IO) during the pandemic【2】.
During the height of the COVID-19 pandemic in 2022, SwiftSwab® quickly gained nationwide presence under emergency authorization, becoming widely available across Canada’s leading pharmacies and supermarkets【3】. It provided millions of households with timely access to reliable at-home testing. Today, with the granting of formal Class IV licenses, SwiftSwab® stands among the first Canadian national brands to secure long-term regulatory approval, transforming from an “emergency testing tool” into a sustainable health management solution.
The Milestone in Context
- National Brand Breakthrough: As a proudly Canadian brand, SwiftSwab® has earned formal Health Canada approval through independent innovation and rigorous quality systems【1】.
- Long-Term Market Compliance: Unlike time-limited emergency authorizations, Class IV licenses ensure SwiftSwab® products can be marketed and distributed sustainably across Canada【1】.
- Technological Edge: The SwiftSwab® Combo test, which simultaneously detects COVID-19 and Influenza A/B, addresses the growing need for multi-pathogen respiratory diagnostics, further reinforcing its competitive advantage【4】.
- Public Health Contribution: With this approval, SwiftSwab® will continue to broaden its reach, supporting pharmacies, hospitals, and Canada’s public health system with early detection and prevention tools【5】.
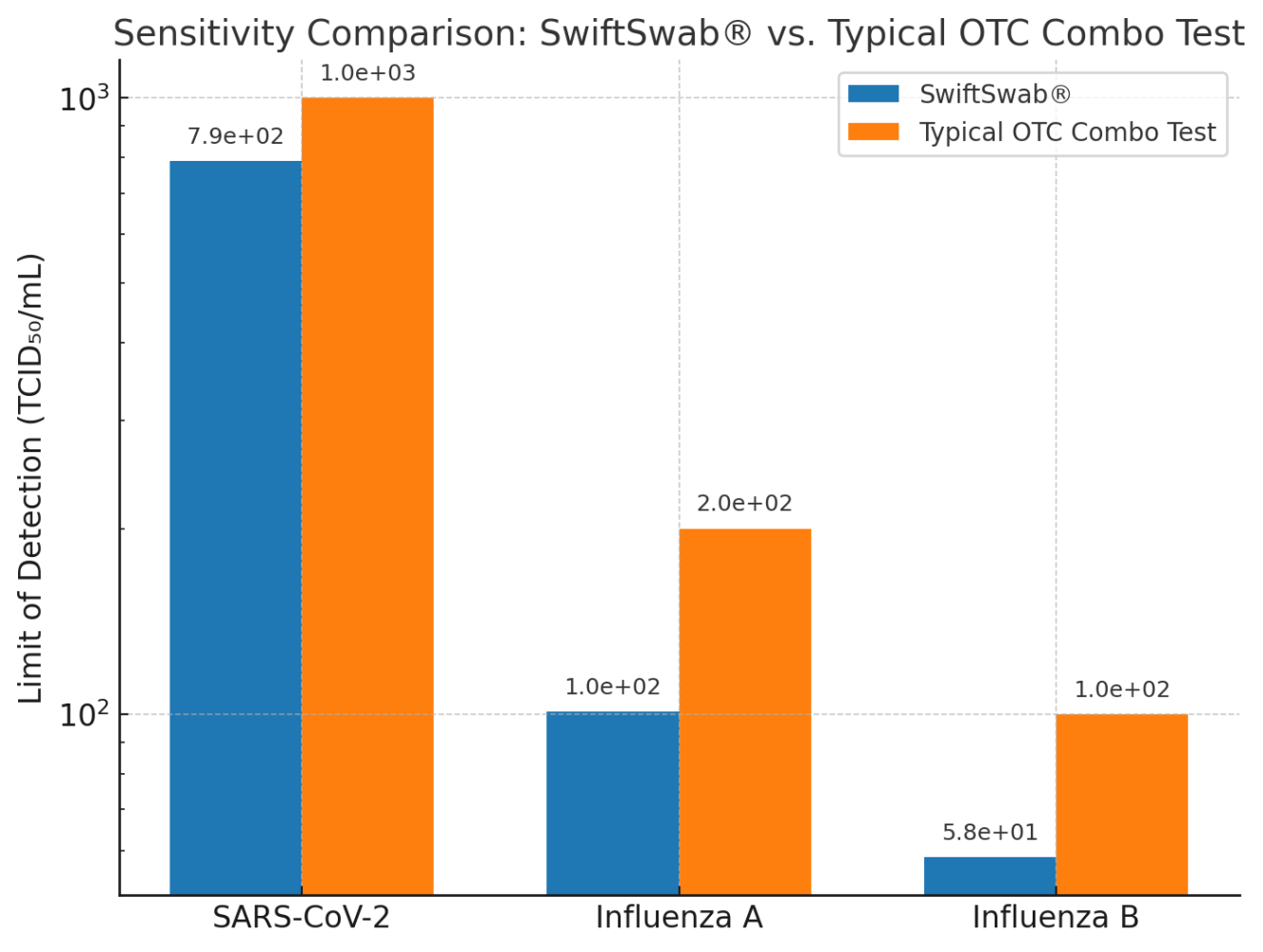
Proven Sensitivity
Independent performance evaluations demonstrate that the SwiftSwab® Combo Home Test delivers robust sensitivity across multiple pathogens. The limit of detection (LOD) was measured at approximately:
- 7.9 × 10² TCID₅₀/mL for SARS-CoV-2
- 1.01 × 10² TCID₅₀/mL for Influenza A
- 5.85 × 10¹ TCID₅₀/mL for Influenza B【6】
These results confirm that SwiftSwab® not only meets but in some cases exceeds international benchmarks for rapid antigen testing【7】, providing consumers with confidence in fast and reliable results from the comfort of their home.
“The SwiftSwab® bar is lower (indicating better sensitivity), with a particularly clear advantage in Influenza B detection.”
Voices of Leadership
“SwiftSwab® was once the brand Canadians picked up on Pharmacy shelves during the pandemic. Today, it is the first Canadian national brand to earn a formal Class IV license from Health Canada. This milestone is more than regulatory recognition – it is proof that Canadian innovation can compete and lead on the global stage,” said Jessica Hu, Founder & CEO of Gene Biotechnology Enterprises Ltd.【8】
Pharmacists’ Endorsement
Since its launch, SwiftSwab® has been widely recommended and praised by pharmacists across Canada, who value its reliability, Canadian origin, and ease of use【9】. This endorsement has cemented SwiftSwab®’s reputation as the go-to trusted brand for at-home rapid testing.
Significance for Canada
This approval not only ensures SwiftSwab®’s long-term presence in the Canadian market but also demonstrates the country’s ability to develop and commercialize homegrown diagnostic solutions【10】. It sets SwiftSwab® apart from foreign-imported brands, positioning it as a flagship Canadian innovation in public health.
About SwiftSwab®
SwiftSwab® is owned by Gene Biotechnology Enterprises Ltd., headquartered in Richmond, British Columbia. The brand is dedicated to developing high-precision, affordable, and user-friendly molecular diagnostics and rapid testing solutions【11】. Having established itself as a trusted household name in Canada with its COVID-19 rapid antigen tests, SwiftSwab® continues to innovate across respiratory diseases, chronic condition management, and public health solutions, reinforcing its role as a flagship Canadian brand in global diagnostics.
Looking Ahead
With Health Canada’s approval, SwiftSwab® will continue to expand its portfolio, including future multi-pathogen rapid tests that go beyond COVID-19 and influenza【12】. The brand remains committed to empowering families, supporting pharmacists, and strengthening Canada’s public health resilience.
References
- Health Canada – Medical Device Licence Listing (MDALL), Licence Nos. 113847 & 113844.
- Government of Canada – Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19.
- Richmond News – “New COVID-19 Rapid Test from Richmond Company Approved in Canada” (Aug 2022).
- FDA Press Release – “FDA Authorizes Marketing of First Home Flu and COVID-19 Combination Test Outside of Emergency Use Authorities” (Oct 7, 2024).
- PHAC – Canadian Respiratory Virus Surveillance Reports.
- Internal Validation Data – SwiftSwab® Combo Limit of Detection Study (2025).
- WHO/FDA Guidelines – Minimum performance requirements for rapid antigen tests (LOD ~10²–10³ TCID₅₀/mL).
- Company Statement – Jessica Hu, Founder & CEO, Gene Biotechnology Enterprises Ltd.
- Pharmacist Testimonials – Canadian Pharmacy Network (2023–2025).
- Asia Pacific Foundation of Canada – “Canadian Biotech Innovation in Diagnostics” Report (2024).
- Gene Biotechnology Enterprises Ltd. – Corporate Profile (2025).
- SwiftSwab® R&D Roadmap – Multi-pathogen POC Diagnostics (2025).