Preparing for the Next Pandemic: The Monkeypox
3 test kits developed by Gene Bio Medical are expected to roll out in soon future
While the COVID-19 pandemic is now largely under control and public-health restrictions are eased in most countries, the unexpected outbreak of monkeypox has thwarted the people’s plan of returning to a normal life. As of mid-June 2022, there were more than 1,600 confirmed cases of monkeypox and nearly 1,500 suspected cases of monkeypox globally, with 150 cases reported in Canada1,2.
Monkeypox, a cousin of the smallpox that shares similar symptoms, is a viral zoonotic disease that can spread from animals to humans and between people. The risk of catching monkeypox from animal hosts (including rodents and primates) can be largely reduced by minimizing unprotected contact with wild animals1,3.
For human-to-human transmission, monkeypox virus is spread through bodily fluids, lesions, and respiratory droplets during physical contact with an infected person, or from mother to fetus. It is also possible to become infected through contact with a symptomatic person’s clothing, bedding, towels, or used utensils1,3.
Common symptoms of monkeypox include fever, intense headache, muscle aches, back pain, low energy, and swollen lymph nodes, followed by development of skin rash 1-3 days after fever onset. Patients may develop skin rash on their face, palms of the hands, soles of the feet, mouth, genital, and eyes3.
The symptoms usually last for 2 to 4 weeks and disappear without treatment. Since the virus can be easily transmitted through physical contact, it is important to protect others by self-isolating when symptoms appear1,3.
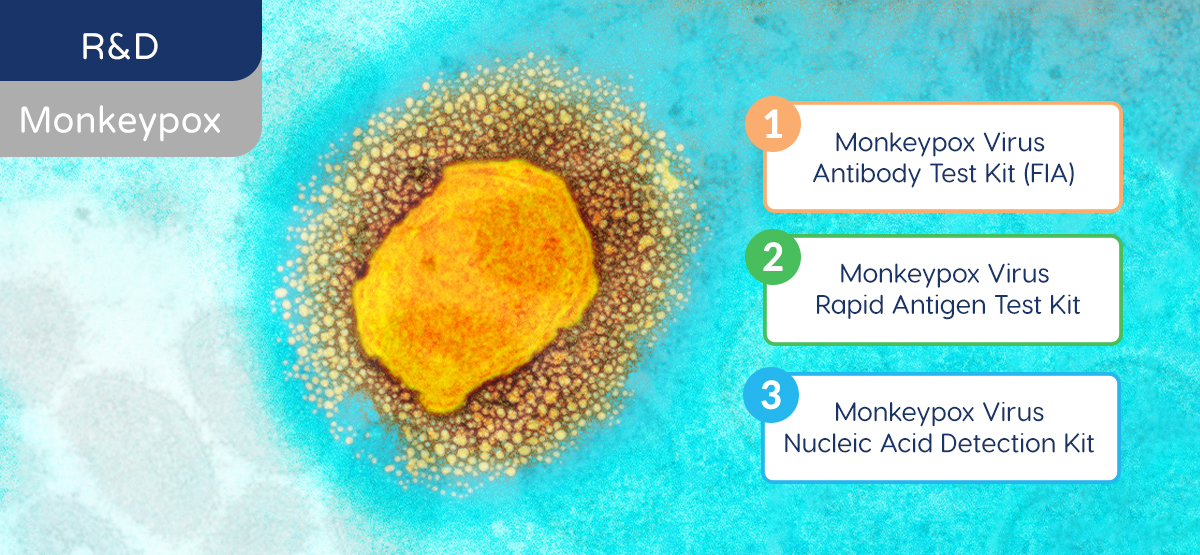
Rapid diagnosis is the key to help people make informed decisions. As a leading biomedical technology company in the field of IVD, Gene Bio Medical is committed to the R&D of efficient IVD diagnostic solutions for monkeypox with 3 test kits in development:
- Monkeypox Antibody Test (FIA): identifies IgM and IgG antibodies produced in response to the monkeypox virus in the blood samples to check whether the individual was previously infected by the virus
- Monkeypox Antigen Rapid Test: identifies the presence of monkeypox-specific antigens in human samples to provide rapid screening of active infection
- Monkeypox Nucleic Acid Detection Kit: using fluorescent PCR method to detect specific viral DNA sequences for early and rapid diagnosis of monkeypox
The test kits are now under review and seeking authorization by Health Canada with expectation to be launched in the near future.
References:
- WHO to determine if Monkeypox should be declared ‘Emergency of International Concern’; rights expert warns of COVID ‘vaccine apartheid’. UN News. Available at https://news.un.org/en/story/2022/06/1120392. Retrieved on June 16, 2022.
- Monkeypox: Outbreak update. Government of Canada. Available at https://www.canada.ca/en/public-health/services/diseases/monkeypox.html. Retrieved on June 16, 2022.
- Monkeypox Q&A. WHO. Available at https://www.who.int/news-room/questions-and-answers/item/monkeypox?gclid=CjwKCAjwqauVBhBGEiwAXOepkQgEQd8iCCP4UzKd5JaZ9i4DfMNERoJvIf4-1ilsRUsHYAq-liBlzxoCKfMQAvD_BwE. Retrieved on June 16, 2022.